Structural changes during the overoxidation of electrochemically deposited poly(3,4-ethylenedioxythiophene) films
The paper authored by
M. Ujvári,
G. G. Láng,
S. Vesztergom,
K. J. Szekeres,
N. Kovács and J. Gubicza
is published in Journal of Electrochemical Science and Engineering (2016, vol. 6, pp. 77–89).
Abstract:
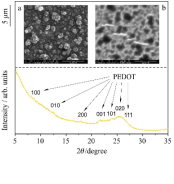
Electrochemical, mechanical and morphological properties of thin poly(3,4-ethylenedioxy-thiophene) (PEDOT) films deposited on gold were investigated in aqueous sulfuric acid and sodium sulphate solutions. At sufficiently positive electrode potentials overoxidation of the polymer took place and resulted in morphological changes and structure evolution. These effects were monitored by electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM) and X-ray diffraction. Significant changes in the film stress caused by overoxidation were detected by using the electrochemical bending beam method. Results of the EIS measurements proved that the charge transfer process at the metal/film interface is more hindered in case of the degraded film. According to SEM images the overoxidation/degradation of PEDOT films can result in random-like but quite well-ordered arrays of islands and trench-like structures. The diffraction peaks of PEDOT became sharper and more intensive during the subsequent oxidation cycles indicating an increase in the degree of crystallinity of the polymer.
